





Acid rain is a form of environmental pollution that occurs when sulfur dioxide (SO2) and nitrogen oxides (NOx) are released into the atmosphere, either naturally from volcanic eruptions or from human activities such as burning fossil fuels (coal, oil, and natural gas) and industrial processes. These pollutants can undergo chemical reactions in the atmosphere and combine with water vapor to form sulfuric acid (H2SO4) and nitric acid (HNO3). When these acidic compounds fall to the Earth’s surface through precipitation, such as rain, snow, fog, or dust particles, it is referred to as acid rain.
The harmful effects of acid rain on aquatic life are particularly pronounced in bodies of water such as lakes, rivers, and streams, as well as in aquatic ecosystems like wetlands and coastal areas. Here are some of the key ways in which acid rain negatively impacts aquatic environments:
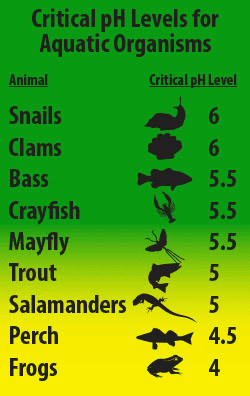
Lowering pH Levels: Acid rain lowers the pH levels of water bodies. The pH scale measures the acidity or alkalinity of a substance, with 7 being neutral. As pH levels decrease below 7, water becomes more acidic. Many aquatic organisms, including fish, insects, and plants, have specific pH ranges in which they thrive. Acidic conditions can disrupt these ecosystems by pushing pH levels outside the tolerance range of native species.
Direct Harm to Aquatic Organisms: Acidic water can directly harm aquatic organisms in several ways. It can damage fish gills, reducing their ability to extract oxygen from the water. Additionally, the aluminum leached from soil and rocks by acid rain can become toxic to fish and other aquatic life. Acid rain can also harm the eggs and larvae of many species, leading to reduced survival rates.
Disruption of Food Chains: Acid rain can impact the availability of food for aquatic organisms by affecting the pH-dependent chemistry of lakes and streams. Algae, which are primary producers at the base of aquatic food chains, may be negatively affected by changes in pH. This can, in turn, disrupt the entire aquatic food web.
Decline in Biodiversity: As certain species are unable to adapt to or survive in more acidic conditions, it can lead to a decline in biodiversity in affected water bodies. Some species may thrive in the altered environment, while others may be severely impacted or driven to extinction.
Changes in Water Chemistry: Acid rain can alter the chemistry of aquatic environments by releasing substances like aluminum and heavy metals from soil and rocks. These substances can accumulate in water bodies, posing additional threats to aquatic life.
Damage to Aquatic Plants: Aquatic plants play a crucial role in stabilizing and oxygenating aquatic ecosystems. Acid rain can damage these plants, which can have cascading effects on the overall health of aquatic environments.
Long-Term Ecosystem Effects: The effects of acid rain can persist over long periods, making it challenging for affected ecosystems to recover even if acid rain levels are reduced. This can lead to chronic stress on aquatic ecosystems.
Efforts to mitigate the harmful effects of acid rain have included the reduction of sulfur dioxide and nitrogen oxide emissions through regulatory measures, such as the Clean Air Act in the United States. Monitoring and research continue to be essential for understanding and addressing the ongoing impact of acid rain on aquatic ecosystems.




